Everything in the Universe — from Earth’s core to farthest galaxies — is made of atoms. It’s a fundamental unit of an element.
So far, 118 elements have been identified (all are listed in the periodic table).
The word ‘atom’, meaning ‘uncuttable’, comes from the ancient Greek word ‘atomos’. Ancient Greek philosophers thought atom is impossible to further divide into anything smaller. However, scientists proved this fact wrong in the early 20th century when they discovered subatomic particles (electrons, protons, and neutrons).
Below, we have listed some of the most intriguing facts about atoms that will only make you smarter. So let’s start with the shortest and simplest one.
Table of Contents
1. Composition of Atoms
Every atom contains one nucleus [at the center] and one or more electrons. The nucleus is typically made of an equal number of protons and neutrons, together called nucleons.
2. Nucleus Contains Almost All Mass
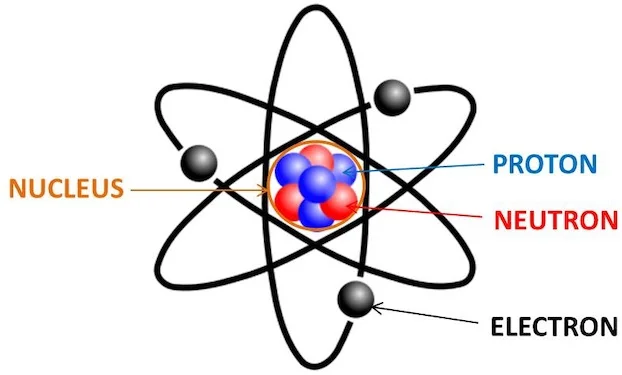
Nucleus, located at the center of the atom, makes up more than 99.95% of its mass but occupies only one trillionth of its total volume. Thus, most of the space inside an atom is empty.
To put this into perspective, if the nucleus was the size of a small marble, the whole atom would be about the size of a football stadium.
3. Electrons Are Extremely Small
Electron is the most active component of an atom, but it contributes almost nothing to the atom’s mass. In the hydrogen atom, for example, the mass of the electron is only 0.0005 times the mass of the nucleus.
4. Atom Can Have Electric Charge
Electrons carry a negative charge, protons carry a positive charge, and neutrons have no electric charge. The atom is electrically neutral if it has the same number of electrons and protons.
However, if an atom has fewer or more protons than electrons, it has an overall positive or negative charge (known as an ion).
5. What Holds Protons And Neutrons Together?
The nuclear force holds protons and neutrons together within the atomic nucleus. The electrons are attracted to the protons by a different force called electromagnetic force, which is weaker than the nuclear force.
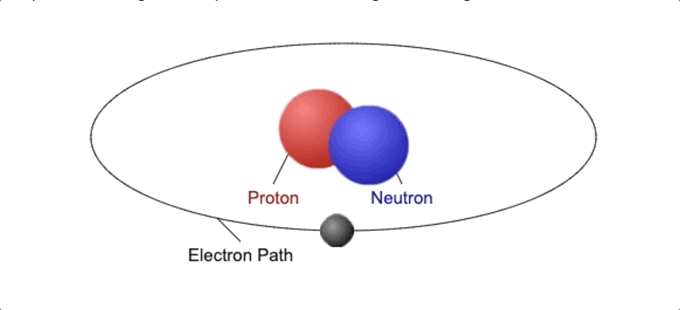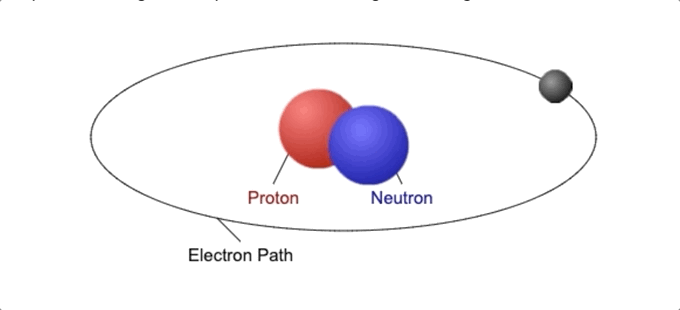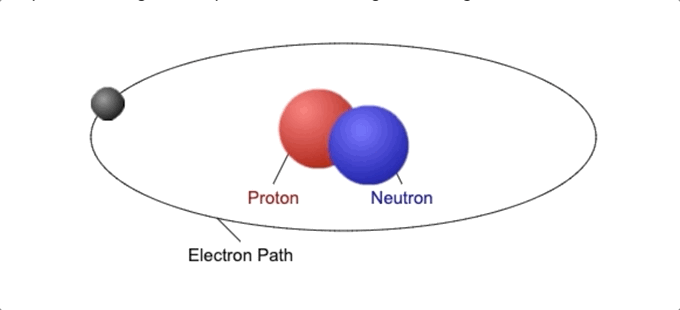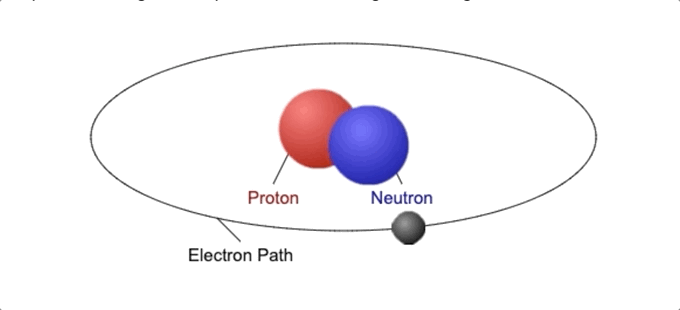
This nuclear force is about 1,038 times stronger than the gravitational force but only operates on a very small scale.
6. 94 Atoms Occur Naturally On Earth
Out of 118 known atoms, 94 occur naturally, though some are found in minute amounts. The remaining 24 have only been synthesized in labs or nuclear reactors.
7. Every Atom Is Unique
Each atom contains a specific number of protons in the nucleus. For example, all sodium atoms contain 11 protons, and all silver atoms contain 47 protons.
The isotope of an element is defined by the number of neutrons, and magnetic characteristics are influenced by the number of electrons in the atom.
8. Biggest And Smallest Atom
The largest element (in terms of size) is Francium, but since it is extremely unstable, the prestige goes to Cesium. It has a big valence shell and a relatively less effective nuclear charge.
 An illustration of the helium atom | Credit: Wikimedia
An illustration of the helium atom | Credit: Wikimedia
The smallest element is Helium, the first in the noble gas group in the periodic table. Its atomic radius is roughly 9 times smaller than the diameter of Cesium.
9. Heaviest and Lightest Atom
Oganesson is the heaviest element (in terms of atomic mass) discovered in 2002. It is the first noble gas that is surprisingly chemically reactive and exhibits very unusual physical and chemical properties.
Oganesson, however, is the heaviest synthetic chemical element. The naturally-occurring heaviest element is Uranium, with an atomic weight of 238.02.
The element that has the lightest atom is Hydrogen. It has only one proton orbited by one electron. Its most common isotope, known as protium, consists of one proton and zero neutrons.
10. Is It Possible To Convert One Element To Another?
Under some extreme conditions, the electromagnetic force (that repels electrons and protons) overcomes the strong nuclear force, ejecting nucleons from the atomic nucleus and leaving behind a whole different element. This is exactly what happens in nuclear fission.
However, this [decay] process is both expensive and dangerous. Scientists haven’t been able to safely generate energy using nuclear fission yet.
Read: Ghost Chemical Bond | A Whole New Perspective Of Bonding Atoms
11. Atoms In Human Body
A human body weighing 155 pounds consists of 7×1027 atoms. Three atoms (hydrogen, oxygen, and carbon) add up to 99 percent of the total.
What’s more interesting is that 98 percent of these atoms are renewed each year, without us even realizing. The fastest-changing molecule is water: almost 50 percent of water molecules in the body are replaced every 8 days.
Moreover, a human hair — 100 nanometers across — consists of one million carbon atoms.
12. How Many Atoms Exists In The Universe?
The observable universe is vast: it spans approximately 93 billion light-years. As per the theoretical estimation, there are 1078 to 1082 atoms in our universe.
It’s not some made-up number. The calculations are based on hard data (what we know so far about the universe). However, there is a massive difference between these estimates, suggesting a significant degree of error. More accurate numbers will be available as we learn more about the cosmos.
13. Radioactive Atoms
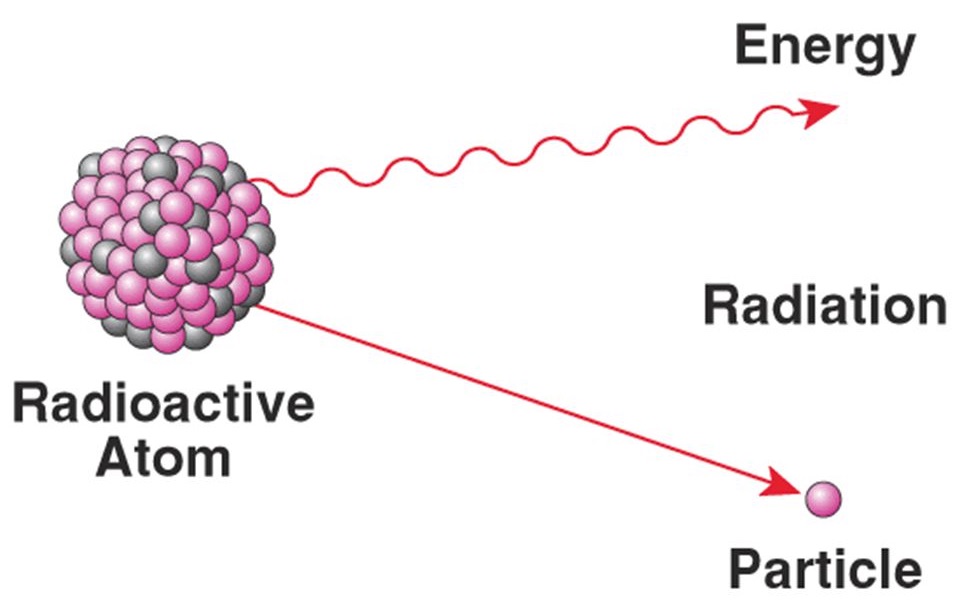
In an unstable atom, the forces are unbalanced. In this case, the atomic nucleus contains an excess of either protons or neutrons. The atom tries to reach a stable state by ejecting its extra particles, or by releasing energy in other forms. Elements containing such unstable nuclei are called radioactive.
Fermium, for example, is a radioactive element: its most stable isotope (Fm-257) has a half-life of 100.5 days.
14. Seeing Atoms
Since atoms are incredibly small as compared to the wavelength of visible light, they cannot be observed even with the world’s strongest optical microscope.
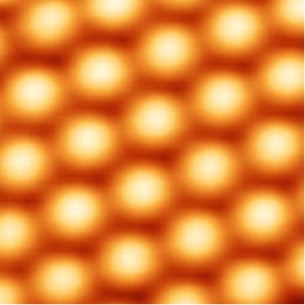 Scanning tunneling microscope captures silicon atoms on the surface of crystal silicon carbide
Scanning tunneling microscope captures silicon atoms on the surface of crystal silicon carbide
That’s why scientists use a different type of microscope, known as a scanning tunneling microscope. It can provide a lateral resolution of 0.1 nanometer and depth resolution of 0.01 nanometer – good enough to image individual atoms within materials.
Read: Measuring The Impossible: Structure of Hydrogen and Helium Atoms Using X-Rays
15. Quantum Nature of Atomic Properties
 Electron making instant ‘quantum leap’ from one energy level to another
Electron making instant ‘quantum leap’ from one energy level to another
Since atoms are extremely small in size, they exhibit quantum properties, thus predicting their behavior by applying classical physics would always lead to incorrect results.
When an electron jumps from one energy level (orbit) to another, it doesn’t move through space between. Instead, it disappears from one orbit and then immediately reappears on another orbit.
Read: Researchers Discovered A Unique Formula Of Pi Hidden In Hydrogen Atoms
In order to better describe and estimate their behavior, several atomic models have incorporated laws of quantum physics.