Chemistry is the science that deals with the structure, composition, and properties of substances. It also includes studying how these substances undergo specific chemical changes and how they release or absorb energy during the transformation.
Since chemistry provides a foundation for understanding basic as well as applied scientific disciplines at a fundamental level, it is often referred to as the central science.
In the 16th and 17th centuries (the era of early chemistry), one person could hope to have a detailed knowledge of all subfields of chemistry. However, science and technology have changed a lot since then. Modern chemistry can be broken into several key subdisciplines that emphasize the subsets of chemical concepts.
Below, I have listed several different types of chemistry that deal with certain aspects of the universe.
Table of Contents
1. Physical Chemistry

Applying pure physics to chemical problems
Physical chemistry involves studying the behavior of substances at different scales, from macroscopic to subatomic levels. Unlike other branches, it primarily deals with the laws of physics that underlie all chemical interactions. The goal is to measure, correlate, and describe the quantitative aspects of reactions.
To understand the nature of atoms and their bonds, it’s important to know how electrons are distributed around their nuclei. These problems are handled by a specific subfield called quantum chemistry. It provides special laws and tools to determine what shape and how strong bonds are, how nuclei move, and how electrons change their shells.
Another important subfield is photochemistry, which studies how light interacts with matter. This is crucial for spectroscopy, a fundamental exploratory tool for determining the chemical composition of a compound.
Since different substances interact with light differently, we can identify them based on how they interact with light. This has helped us understand the composition of distant celestial bodies, such as planets, comets, and asteroids.
Physical chemists use sophisticated equipment such as lasers, electron microscopes, nuclear magnetic resonance, etc. to analyze substances, develop techniques to test can characterize the properties of substances, develop theories about these substances, and discover their potential applications. They may also apply mathematical analysis on massive datasets and conduct simulations to predict how these substances will react over time.
2. Organic Chemistry

Studying molecules containing carbon and hydrogen
In organic chemistry, we learn about compounds that contain covalently bonded carbon atoms. Unlike other atoms, carbon has the unique ability to form chains with other carbon atoms and various elements such as nitrogen, oxygen, halogens, sulfur, and many more. It can form millions of organic compounds that may consist of any number of other elements.
This field of chemistry primarily concerns the chemical composition, structure, and physical properties of organic compounds. It also involves evaluating the chemical reactivity of organic compounds to understand their behavior.
Organic chemistry plays a crucial role in the development of common household chemicals, foods, and fuels. Advancements in this field have made several contributions to our society, such as the synthesis of polymers (which includes all plastics and rubbers), numerous medicines, and other useful compounds like ethanol and insulin.
Because all known life is based on organic compounds, many careers require an understanding of organic chemistry, including pharmacologists, dentists, chemists, doctors, veterinarians, and chemical engineers.
3. Inorganic Chemistry
 Image credit: libretexts.org
Image credit: libretexts.org
Covers non-carbon-based compounds
Inorganic chemistry deals with compounds that lack carbon-hydrogen bonds. On Earth, there are about 100,000 known inorganic compounds (in contrast to 2 million inorganic compounds). This field aims to study the structure, composition, and behavior of these compounds.
Some common examples of inorganic compounds include silicon dioxide (used in solar cells and computer chips), sulfuric acid (used in fertilizers and household products), and sodium chloride (used as table salt). They all can be classified as bases, acids, oxides, and salts.
Synthesis of inorganic chemicals involves transforming raw materials and compounds. They can undergo four types of chemical reactions: combination, decomposition, single-displacement, and double-displacement reactions.
One of its rapidly growing subfields is organometallic chemistry, which bridges the gap between organic and inorganic chemistry. It covers compounds containing at least one bond between a metal atom and a carbon atom. Organometallic compounds are widely used in research and as catalysts to accelerate chemical reactions, especially when the target molecules are pharmaceuticals or polymers.
Overall, inorganic substances have a substantial impact on the world economy. They are used in various industrial processes and products, including pigmentation, surfactants, catalysis, coating, medicine, materials science, and electronic devices.
4. Analytical Chemistry
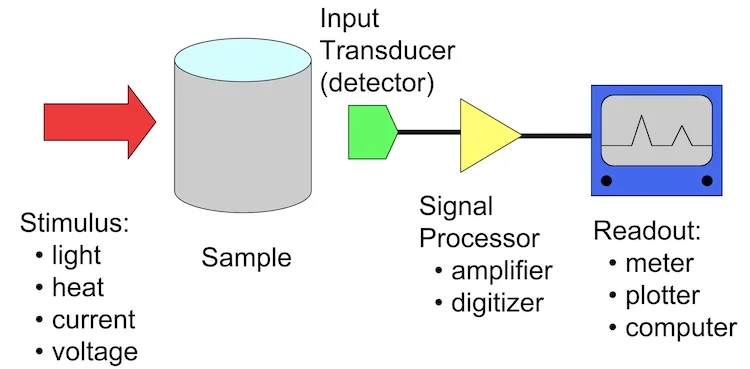 Working principle of a typical analytical instrument
Working principle of a typical analytical instrument
Science of identifying and quantifying the data about the structure and composition of matter
Analytical chemistry uses advanced techniques and instrumentation to isolate specific compounds, identify those compounds, and determine the quantity of the compounds in a product.
It can be further divided into two fields: quantitative analysis and qualitative analysis. The former is used to determine the absolute value or relative quantity of one or more substances present in a compound. The latter deals with the determination of the quality of a specific compound, irrespective of its concentration or quantity.
For example, detecting iron in magnetite is a qualitative analysis, whereas measuring the actual amount of iron (72.3% by mass) in magnetite is a quantitative analysis.
Analytical chemistry is used in various areas of science. For example, it can be used to identify unknown substances at a crime scene, determine cholesterol levels in a blood sample, purify engine oil, and much more. It has major applications in bioanalysis, clinical analysis, forensic science, materials analysis, and environmental analysis.
5. Nuclear Chemistry
 Atomic nucleus emitting an alpha particle
Atomic nucleus emitting an alpha particle
Studying changes in the nuclei of atoms
Nuclear chemistry is concerned with changes in the nucleus of elements, which are the source of nuclear power and radioactivity. Some elements on Earth are radioactive. They spontaneously emit radiation (such as alpha, beta, or gamma radiation).
Unlike conventional chemical reactions that form compounds, nuclear reactions transform one element into another. This property is utilized in nuclear power plants to collect and store nuclear energy.
Modern nuclear chemistry (often called radiochemistry) has a wide range of applications, from designing radioactive methods for diagnostic medicine to studying the formation of elements in the universe.
In fact, the advances made by nuclear chemists have become so important that physicists, geologists, and biologists use nuclear chemistry as a regular tool in their disciplines.
A combination of radiation chemistry and radiochemistry is used to analyze nuclear reactions such as fusion and fission. Nuclear fusion, in particular, emits enormous amounts of energy and is generally referred to as a thermonuclear reaction.
The Sun and other stars in the universe are actually giant fusion reactors. In these stars, hydrogen molecules (under tremendous pressure of gravitational forces) are fused into helium and heavier elements. During the reaction, massive energy is released in the form of light and heat.
Nuclear chemistry also covers the nuclear processes in non-radioactive areas of human activity. Nuclear magnetic resonance spectroscopy, for example, is widely used in physical chemistry, synthetic organic chemistry, and macromolecular chemistry.
6. Biochemistry

Explores chemical processes that occur in or are related to living organisms
Biochemistry involves studying the chemical substances and processes that occur in animals, plants, and microorganisms, and the changes they undergo throughout their lifetimes.
Basically, it’s a laboratory-based science that brings chemistry and biology together. It focuses on what happens inside living cells and how they communicate with each other during growth or fighting illness. It primarily deals with the structures, functions, and interactions of biological macromolecules, such as carbohydrates, lipids, nucleic acids, and proteins.
Although biochemistry is still a young science, having been known by that name only since the late 19th century, it has successfully explained living processes through structural biology, enzymology, and metabolism.
Biochemistry also describes the tools and techniques required to understand the functioning of biological molecules. This includes traditional techniques like chromatography, western blotting, and co-immunoprecipitation analysis.
Overall, it overlaps with a range of scientific disciplines, including microbiology, genetics, medicine, plant science, and forensics.
Read: 17 Best Science And Technology Research Labs In The World
Other Emerging Subfields
7. Computational Chemistry

Utilizes computer simulation to solve complex chemical problems
As the name suggests, computational chemistry uses computer simulation to calculate the structures and properties of compounds or groups of molecules. Although it’s not an exact description of real-life chemistry, chemical phenomena can be explained to some extent using an approximate quantitative or qualitative computational scheme.
Chemical scientists and engineers exploit advances in computing hardware and software, as well as in new theoretical and mathematical approaches. Most discoveries are based on the use of massively parallel high-performance CPUs and GPUs to solve complex equations.
For example, simulations and calculations done on supercomputers have improved our understanding of copper-catalyzed cyclopropanation, zinc-catalyzed alkylation, rhodium-catalyzed hydrogenation, the origin of enantioselectivity in transition metal-catalyzed asymmetric synthesis, and various other processes.
8. Quantum Chemistry
Applying quantum mechanics to chemical systems
In simple terms, quantum chemistry is the study of very small particles. The field emerged with the discovery of subatomic particles — electrons, protons, and neutrons.
One of the main goals of quantum chemistry is to understand the electronic structure and molecular dynamics using the Schrödinger equations. In 1926, Erwin Schrödinger developed a mathematical equation that shows if you know the potential energy acting on an object, you can measure the wavefunction for the object. And once you have the wavefunction, you can determine the properties of that object.
However, the exact solution for Schrödinger’s wave equation cannot be obtained for larger atoms and molecules (containing more than one electron). Quantum chemistry seeks to simplify assumptions/approximations and to increase the accuracy of solutions for small and large molecular systems.
Recent developments in quantum-mechanical modeling methods, such as density functional theory, have enabled accuracies comparable to those achieved in experiments on moderate-sized molecules.
9. Astrochemistry

Study of molecules in space and their interaction with radiation
Astrochemistry is the science that studies the chemical composition of matter in space and the processes that led to those compositions. It is applied to both the Solar System and the Interstellar medium.
Astrochemists, who are part astronomers and part chemists, analyze molecules and ions in outer space to determine their roles in the composition of the universe. This includes the atoms and molecules that make up the gaseous matter of future asteroids, stars, and even entire solar systems.
They utilize different types of radio telescopes to detect electromagnetic radiation emitted by celestial bodies. Once you know the frequency of waves (radio, gamma, ultraviolet, or infrared waves), you can determine what molecules are in space in what quantities. The data is then merged with information from other fields, such as astrophysics and meteorology, to better understand the origin of our universe.
10. Phytochemistry

Study of chemicals derived from plants
Phytochemistry deals with the chemical processes associated with plant life and the chemical compounds produced by plants. Its primary goal is to study phytochemicals — bioactive nutrient plant chemicals in vegetables, grains, fruits, and other plant foods that may provide health benefits beyond standard nutrition.
Phytochemicals are used in soft drinks, functional foods, and numerous other food products that offer good nutritional value and significant importance. Flavonoids, isoflavonoids, phytosterols, glucosinolates, limonoids, and polyphenols are some of the most common phytochemicals that provide substantial health benefits.
Phytochemists try to determine the structures of various secondary metabolites found in plants. They also study how these compounds function in plant and human biology.
There are many different types of compounds found in plants. Most of them can be grouped into four biosynthetic classes: terpenoids, polyketides, phenylpropanoids, and alkaloids.
11. Green Chemistry

Minimizing the use and production of hazardous/unwanted chemical processes and substances
Green chemistry is primarily concerned with the optimization and development of chemical processes and products that aim to reduce (or eliminate) the toxic substances released into the environment.
Unlike environmental chemistry, which focuses on the harmful effects of polluting chemicals on the environment, green chemistry focuses on lowering the consumption of nonrenewable resources and developing new techniques to prevent pollution.
In 1998, Paul Anastas (one of the founders of green chemistry) published 12 principles that address ways to minimize the health and environmental impacts of chemical production. These principles are:
- Prevent waste
- Design processes that utilize the maximum amount of raw materials
- Avoid using toxic chemicals
- Design safer chemicals
- Design safer solvents and auxiliaries
- Make the chemical process energy efficient
- Use renewable feedstocks
- Minimize unnecessary production of derivatives
- Use non-toxic catalysts
- Use products that can be broken down into non-harmful substances
- Monitor the whole process in realtime so it can be halted before the formation of hazardous substances
- Minimize risks of explosions, fires, and accidental releases
Although these principles are not new, the extent to which they are being applied has led to intensified attention on this topic among the industrial, academic, and regulatory communities.
Read: 8 Different Types Of Research [With Examples]
Frequently Asked Questions?
What are the major types of chemical reactions?
Many types of chemical reactions occur in nature, but most can be divided into six groups: combination reactions, decomposition reactions, neutralization reactions, combustion reactions, displacement reactions, and precipitation reactions.
How many types of chemical bonds are there?
In chemistry, there are four primary types of bonding:
- Covalent bonds: Atoms bonded by sharing electrons
- Hydrogen bonds: interaction involving a hydrogen atom located between a pair of highly electronegative atoms
- Ionic bonds: Electrostatic attraction between oppositely charged ions
- van der Waals interactions: Intermolecular interactions that do not contain ions or covalent bonds
Who is known as the father of chemistry?
Jabir ibn Hayyan is known as the father of early chemistry. He introduced a systematic classification of chemical substances. He also figured out a way to obtain an inorganic compound (ammonium chloride) from organic substances (like blood, hair, and plants) by chemical means.
Read: 13 Different Types Of Scientists
The title of father of modern chemistry goes to French chemist Antoine Lavoisier. In 1778, he recognized and named oxygen and explained its role in combustion. Later, he established that water is a compound and not an element.
Lavoisier created the first extensive list of elements and helped to improve chemical nomenclature. In 1787, he estimated the existence of silicon. He also proved that matter could change its shape or form, but its mass always remains the same.